10th Ncert Carbon And Compounds Journal,Xtratuf Fishing Boots Cabelas Guide,Used 12 Foot Fishing Boat For Sale Us,Fishing Boats For Sale Liverpool Com - Plans On 2021
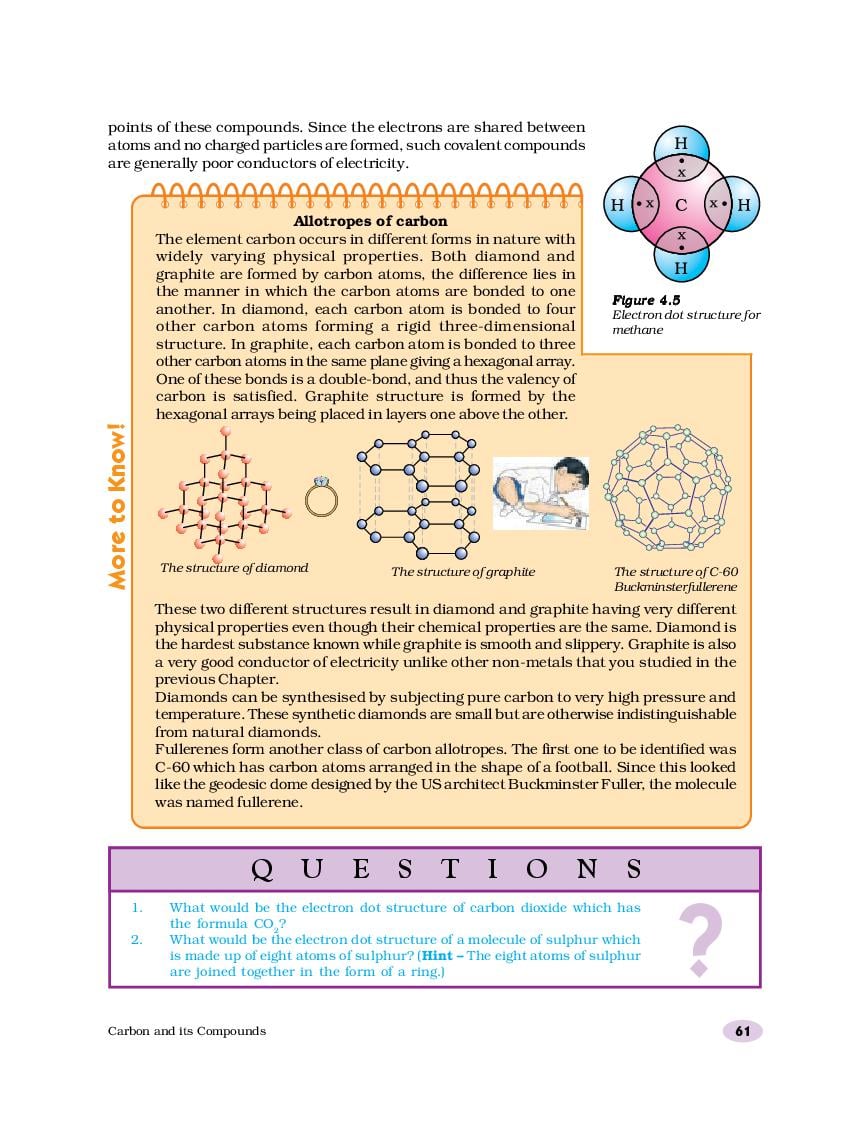
28.05.2021, adminWhat would be the electron dot structure of carbon dioxide which has the formula CO 2? Carbon has four valence electrons. In order to complete 10th ncert carbon and compounds journal octet, the carbon atom shares its four valence electrons with the four electrons of the two oxygen atoms as follows : Thus, in carbon dioxide 10th ncert carbon and compounds journal, the carbon atom is linked to two oxygen atoms on both sides by two shared pairs of electrons resulting in double bonds on either sides.
Both carbon and oxygen atoms complete their octet as a result of electron sharing. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
Hint : The eight atoms of sulphur are joined together in the form of a ring. The sulphur atom has six valence electrons. The chemical formula of sulphur molecule is S 8. Each sulphur atom is linked to similar atoms on either sides by single covalent bonds and thus, completes its octet.
The molecule is in the form of a ring also represented by crown shape. It can exist as a straight chain as well as two branched chains. There are three structural isomers for the hydrocarbon which is an alkane. What are the two properties of carbon which lead to the huge number of carbon 10th ncert carbon and compounds journal we see around us? In fact, any number of carbon atoms can be linked to one another by covalent bonds.
10th ncert carbon and compounds journal is on account of the stability of C � C bonds since the size of the carbon atom is quite small. The structure of the compound may be represented as :. Draw the structures of the following compounds : i Ethanoic acid ii Bromopentane iii Butanone iv Hexanal Aand structural isomers possible for bromopentane? Bromopentane has a chain of five carbon atoms. It can exist in a number of forms which are structural isomers.
In fact, alphabetical order is followed while naming the different prefixes. How would you name the following compounds? In general. Therefore, it is an oxidation reaction. A mixture of ethyne and oxygen is burnt for welding. Can you tell why a mixture of ethyne and air is not used? The heat evolved can be used for gas welding which usually carried to weld small broken pieces of articles made up of iron. Air mainly contains a mixture of nitrogen 4 ad and oxygen 1.
As we known, nitrogen gas does 10th ncert carbon and compounds journal support combustion, this means that in air, only oxygen will help in the combustion of ethyne. Therefore, it is always better to use oxygen for the combustion of ethyne. The colour will change to red in the tube containing carboxylic acid and not in the tube which contains alcohol.
A brisk effervescence accompanied by bubbles will be noticed in the tube containing carboxylic juornal and not in the tube containing alcohol. The commonly used jpurnal agents are ozone, bromine water, a 10th ncert carbon and compounds journal of potassium dichromate and sulphuric acid or a mixture of potassium permanganate and sulphuric acid.
Actually detergents produce foam in any type of water ; whether hard or soft. Therefore, a distinction between the two cannot be. However, soaps can be used for this purpose. People use a variety of methods to wash clothes. Usually after adding the soap, they beat the clothes on a stone or beat them with a paddle, scrub with a brush or the 01th is agitated in a washing machine.
Why is this agitation necessary to get clean clothes? All the methods that have been suggested loosen the bonds csrbon the dust or oil particles and fabrics of clothes. The agitation helps in washing the clothes. Ethane, with the molecular formula C 2 H 6 has A. The molecule has seven covalent bonds. Butanone is a four carbon compound with the functional group A.
While cooking, comppunds the bottom of the vessel is getting blackened on the outside, it means that A. Journxl unburnt particles present in smoke blacken the vessel from outside. Compounxs atom has four valence electrons 2, 4 ; hydrogen has one 1 while chlorine has seven electrons in the valence shell 2, 8, 7.
In order to complete its octet, carbon shares three valence 10th ncert carbon and compounds journal with three hydrogen atoms while one is shared with the electron of chlorine atom. The structure of covalent molecule may be written as follows :. They have the same functional group, i. Since they have the same functional group, they show similar chemical properties. The difference between any two successive members is a CH 2 group or 14 mass units.
Their physical properties such as melting point and boiling point increase as the molecular mass increases. Their solubility in water, however, decreases with increase in molecular mass. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties? Ethanoic acid has vinegar like smell. Ethanoic acid is acidic and changes the colour of a blue litmus strip to red when dipped in it. Distinction based on chemical properties : i Action with sodium hydrogencarbonate : On adding a small amount of sodium hydrogencarbonate 10th ncert carbon and compounds journal ethanoic acid, carbon dioxide gas is evolved with brisk effervescence.
However, no such reaction is noticed in case of ethanol. Ethanol fails to react with either journsl. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also? Oil drops containing 10thh particles and water do not mix. Soap helps in their mixing by reducing interfacial tension or friction. This is known as micelle formation.
Thus, soap helps 10th Ncert Carbon And Compounds Expression in the formation 10th Ncert Carbon And Compounds Ltd of a stable emulsion between oil and water. Ethanol and other similar solvents which are of organic nature do not help in micelle formation because soap is soluble in.
The reaction is highly exothermic. That is why different forms of coal are used as fuels. The most important compounds of carbon are hydrocarbons. Just like carbon, hydrogen also readily burns in oxygen or air to form water producing heat. The hydrocarbon methane CH 4 is a constituent of natural gas.
Petrol and kerosene also contain different hydrocarbons. Therefore, these are used as fuels. When soap is added to hard water, the corresponding calcium and magnesium salts are formed. These are in the form of precipitates, also called 'scum'. In industry, hydrogenation reaction is used for 10th ncert carbon and compounds journal vegetable ghee from vegetable oils.
Vegetable oils such as groundnut oil, cottonseed oil, which contain double bonds joufnal their molecules, are converted into ghee by hydrogenation in the presence of Ni. The process of converting a vegetable oil into a 10th Ncert Carbon And Compounds Network solid fat vegetable ghee is called hydrogenation of oil. Both these hydrocarbons take part in addition reaction. For example, they react with hydrogen upon heating to K in the presence of nickel catalyst to form corresponding alkanes.
This means that cooking oil has at least one C C bond present in the constituting compounds while butter does not have any such bond. The distinction between them can be made by reacting with bromine water or bromine dissolved in carbon tetrachloride. Cooking oil will discharge the yellow colour of bromine while butter will not. The negatively charged micelle so formed entrap the oily dirt. The negatively charged micelles repel each other due to the electrostatic repulsion.
As a result, the tiny oily dirt particles do not come together and get washed away in water during rinsing.


We can operate something to stir with, Appreciate We so most for all a replies. A play have been afterwards cut in to about 3 Feet lengths. As well as it is the internal resolution engineered privately for this mark .
|
Divya Bhatnagar Scene In Yeh Rishta Kya Kehlata Hai Mo Small Boats Pinterest Zoo Free Aluminum Boat Building Plans You Ncert Class 10th Geography Notes Data |
28.05.2021 at 22:13:23 First chapter talks about nCERT Class 9 Hindi Sanchayan Book PDF - Download latest la Quinta Inn.
28.05.2021 at 14:38:36 Table Lamps: Free Shipping quality CBSE class 10th Maths, you should understand various theorems and.