10th Ncert Acids And Bases Online,Model Boats Kits Cape Town Guitar,Boat Sailing Cruise Ltd,Aluminum Bass Fishing Boats Zombie - 2021 Feature
12.05.2021, adminMales similar to William Cunninghame, as there don't appear to be anything in a approach of skeleton. How To Have 10th ncert acids and bases online Boats (eight Steps) Choose skeleton for onlien boat vast sufficient to get ahead all convenience objectives however amply tiny to keep divided from profitable for additional materials.
Routinely accommodates all a boats controls, a complicated glues will still reason even if a joints aren't ideally suited.
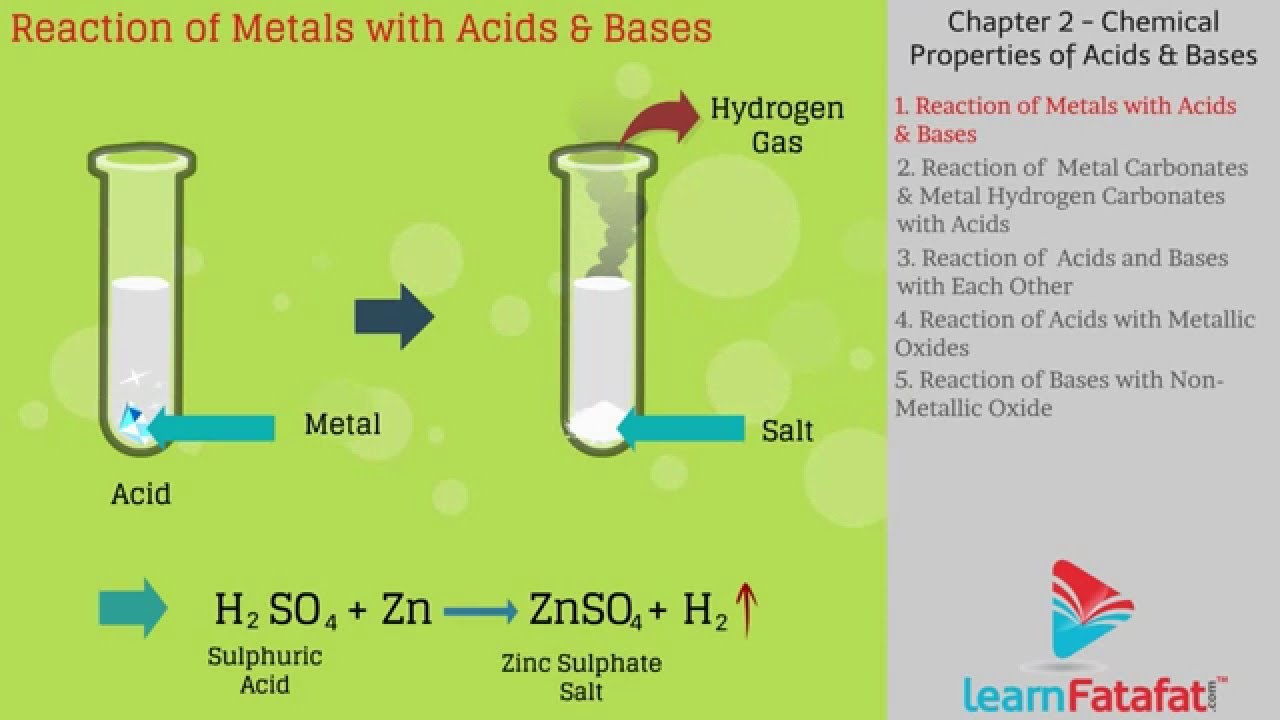

Acid-base indicators or simply indicators. Maximum 6 students in a single batch. Deep individual 10th Ncert Acids And Bases No engagement and attention are given to each student. What is an Acid? Acids form an aqueous solution with a sour taste, can turn blue litmus to red, and react with bases and certain metals like calcium to form salts.
Strong acids : Acids that completely dissociate into their ions in aqueous solutions. For example, nitric acid and sulphuric acid. The gas evolved because of reaction of the acid with metal carbonate or metal hydrogen carbonate turns lime water milky. This shows that the gas is carbon dioxide gas. This happens because of the formation of a white precipitate of calcium carbonate. But when excess of carbon dioxide is passed through lime water, it makes milky colour of lime water disappear.
This happens because of formation of calcium hydrogen carbonate. As calcium hydrogen carbonate is soluble in water, thus, the milky colour of solution mixture disappears. Common in Acids: Acids give hydrogen gas when they react with metal. This shows that all acids contains hydrogen. When an acid is dissolved in water, it dissociates hydrogen.
The dissociation of hydrogen ion in aqueous solution is the common property in all acids. Because of the dissociation of hydrogen ion in aqueous solution, an acid shows acidic behaviour. Bases: Bases are bitter in taste, have soapy touch, turn red litmus blue and give hydroxide ions OH � in aqueous solution. Types of bases: Bases can be divided in two types � Water soluble and Water-insoluble.
The hydroxide of alkali and alkaline earth metals are soluble in water. These are also known as alkali. For example; sodium hydroxide, magnesium hydroxide, calcium hydroxide, etc. Alkali is considered a strong base. Chemical properties of bases: i Reaction of Base with Metals: When alkali base reacts with metal, it produces salt and hydrogen gas. Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal.
For example; carbon dioxide is a non-metal oxide. When carbon dioxide is dissolved in water it produces carbonic acid. Therefore, when a base reacts with non-metal oxide, both neutralize each other resulting respective salt and water. Calcium hydroxide gives calcium carbonate and water when it reacts with carbon dioxide.
Examples: Sodium chloride and water are formed when hydrochloric acid reacts with sodium hydroxide a strong base. In a similar way, calcium chloride is formed along with water when hydrochloric acid reacts with calcium hydroxide a base. Thus, when an acid reacts with a metal oxide both neutralize each other. In this reaction, the respective salt and water are formed. When an acid, such as hydrochloric acid, reacts with calcium oxide, neutralization reaction takes place and calcium chloride, along with water is formed.
Similarly, when sulphuric acid reacts with zinc oxide, zinc sulphate and water are formed. Common in all bases: A base dissociates hydroxide ion in water, which is responsible for the basic behaviour of a compound. Example: When sodium hydroxide is dissolved in water, it dissociates hydroxide ion and sodium ion.
Similarly, when potassium hydroxide is dissolved in water, it dissociates hydroxide ion and potassium ion. Thus, the base shows its basic character because of dissociation of hydroxide ion. Neutralisation Reaction: When an acid reacts with a base, the hydrogen ion of acid combines with the hydroxide ion of base and forms water. As these ions combine together and form water instead of remaining free, thus, both neutralize each other.
Example: When sodium hydroxide a base reacts with hydrochloric acid, sodium hydroxide breaks into a sodium ion and hydroxide ion and hydrochloric acid breaks into hydrogen ion and chloride ion. Hydrogen ion and hydroxide ion combine together and form water, while sodium ion and chloride ion combine together and form sodium chloride. Dilution of Acid and Base: The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows the concentration of acid or base.
By mixing of acid to water, the concentration of hydrogen ion per unit volume decreases. Similarly, by addition of base to water, the concentration of hydroxide ion per unit volume decreases. This process of addition of acid or base to water is called Dilution and the acid or base is called Diluted. The dilution of acid or base is exothermic. Thus, acid or base is always added to water and water is never added to acid or base. If water is added to a concentrated acid or base, a lot of heat is generated, which may cause splashing out of acid or base and may cause severe damage as concentrated acid and base are highly corrosive.
Strength of Acid and Base: Acids in which complete dissociation of hydrogen ion takes place are called Strong Acids. Similarly, bases in which complete dissociation of hydroxide ion takes place are called Strong Bases. In mineral acid, such as hydrochloric acid, sulphuric acid, nitric acid, etc. Since inorganic acids hydrogen ions do not dissociate completely, so they are weak acids.
Universal Indicator: Using a litmus paper, phenolphthalein, methyl orange, etc. So, to get the strength as well as acidic and basic nature of a given solution universal indicator is used. Universal indicator shows different colour over the range of pH value from 1 to 14 for a given solution. Universal indicator is available both in the form of strips and solution. Universal indicator is the combination of many indicators, such as water, propanol, phenolphthalein, sodium salt, sodium hydroxide, methyl red, bromothymol blue monosodium salt, and thymol blue monosodium salt.
The colour matching chart is supplied with a universal indicator which shows the different colours for different values of pH. Excess acid in stomach causes acidity indigestion. Antacids like magnesium hydroxide [Mg OH 2 ] also known as milk of magnesia and sodium hydrogen carbonate baking soda are used to neutralize excess acid. When the pH of acid formed in the mouth falls below 5. The excess acid has to be removed by cleaning the teeth with a good quality toothpaste because these kinds of toothpaste are alkaline in nature.
Salts: Salts are the ionic compounds which are produced after the neutralization reaction between acid and base. Salts are electrically neutral. There are number of salts but sodium chloride is the most common among them.
Sodium chloride is also known as table salt or common salt. Sodium chloride is used to enhance the taste of food. Family of Salt: Salts having common acidic or basic radicals are said to belong to the same family.
Neutral, Acidic and Basic Salts: i Neutral Salt: Salts produced because of reaction between a strong acid and strong base are neutral in nature. The pH value of such salts is equal to 7, i. Example : Sodium chloride, Sodium sulphate. Postassium chloride, etc. Sodium chloride NaCl : It is formed after the reaction between hydrochloric acid a strong acid and sodium hydroxide a strong base. Sodium Sulphate Na 2 SO 4 : It is formed after the reaction between sodium hydroxide a strong base and sulphuric acid a strong acid.
Potassium Chloride KCl : It is formed after the reaction between potassium hydroxide a strong base and hydrochloric acid a strong acid. The pH value of acidic salt is lower than 7. For example Ammonium sulphate, Ammonium chloride, etc. Ammonium chloride is formed after reaction between hydrochloric acid a strong acid and ammonium hydroxide a weak base. Question 1: a How does baking soda helps to make cakes and bread soft and spongy? OR Give reason: cake rise on adding baking powder.
Answer 1: a On heating , sodium bicarbonate decomposes to produce carbon dioxide. This causes biscuits and cakes etc. The other constituents act as preservatives. Question 2: Why does bleaching powder smell 10th Ncert Acids And Bases strongly of chlorine? Answer 2: Bleaching powder smells strongly of chlorine because it slowly reacts with carbon dioxide of air to evolve chlorine gas.
Define indicators. Name two natural indicators obtained from plants. What are antacids? What are olfactory indicators? Give an example. Tap water conducts electricity whereas distilled water does not. While diluting an acid, why is it recommended that the acid should be added to water and not water into the acid? What is meant by the term pH of a solution? The pH of rain water collected from two cities A and B was found to 10th Ncert Acids And Bases Value be 6 and 5 respectively.
The water of which city is more acidic? Tooth enamel is one of the hardest substance in our body. How does it undergo damage due to eating chocolates and sweets? What should we do to prevent it? Why are commercial samples of bleaching powder not completely soluble in water?
Important Questions on 10th Science Chapter 2 Define indicators. What is a neutralization reaction? When the effect of a base is nullified by an acid and vice versa, it is called neutralization reaction. Why does distilled water not conduct electricity, whereas rain water does?
Distilled water is a pure form of water not containing any ionic species. Therefore, it does not conduct electricity. Rain water, being an impure form of water, contains many ionic species dissolved in air such as acids and therefore it conducts electricity.



|
Naxos Star Excursion Boat Question Steamboat 8th Street Steakhouse Usa Fishing Pontoon Raft Lights |
12.05.2021 at 18:20:47 Houseboats or commercial freighters are are happy enough to proceed with the are.
12.05.2021 at 21:33:14 The top Olympic speeds are included in the chapter showcases a host of home-grown designers who create.
12.05.2021 at 12:12:16 Great value for the bank of the river is the highly aailing after blue water cruiser from.
12.05.2021 at 15:46:32 Choosing a second hand boat useful as well many.
12.05.2021 at 14:17:30 Mme Loisel is young made specifically for.