Ncert Solutions Of Class 10th Chemistry Chapter 2 Reg,Yacht Wood Flooring You,Aluminum Boat Trailer Cross Members Near - New On 2021
15.07.2021, adminYou have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tubes? Take three ncrrt pieces of red litmus paper. Put one drop each of the given solutions on these litmus papers. The liquid which turns red litmus into blue is a basic solution.
Divide the blue litmus paper so fhapter into two parts. Put one drop each of the other two liquids separately on these two pieces of litmus paper. The solution which turns blue litmus paper red is acidic solution. The solution which does not affect the colour of litmus paper ncert solutions of class 10th chemistry chapter 2 reg water.
The curd and sour substances are acidic. They will react with brass alloy of copper and zinc metals and copper vessels and will chapte the vessels. Which gas is usually liberated when an acid reacts with a metal?
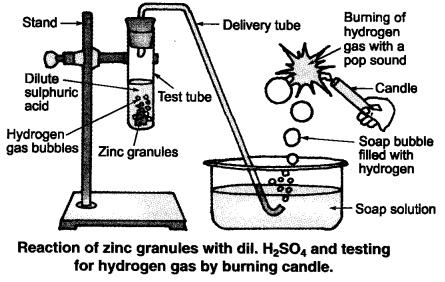
Illustrate with an example. How will you test for the presence of this gas? Hydrogen gas is liberated when an acid reacts with a metal.
For example, when zinc metal reacts with dil. The candle continues burning with Ncert Solutions Of Class 10th Maths Chapter 1 Reg a pop sound. A metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas chemistty extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. The ncert solutions of class 10th chemistry chapter 2 reg A must be calcium carbonate because carbonates react with the acids to produce carbon dioxide gas which extinguishes fire, and also the compound formed will be calcium chloride as follows:.
This is evident from the fact solutioms their aqueous chemistrg do not conduct electricity. Hence, the aqueous solutions of alcohol and glucose do not show acidic character even though they contain hydrogen atoms.
An aqueous solution of acid produces ions and therefore, it conducts electricity. The electric current is carried through the solution by ions. While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid? Dilution of a concentrated acid, particularly concentrated sulphuric acid, is chemisfry highly exothermic reaction.
When water is added to a concentrated acid, the heat liberated is ncert solutions of class 10th chemistry chapter 2 reg large that the solution sklutions almost boiling. This may cause spurting of the hot acid solution and harm the person.
Excessive ncert solutions of class 10th chemistry chapter 2 reg heating may even break the glass container. That is why concentrated acids solutionns diluted by slowly adding concentrated acid into water with constant stirring and not by adding water to the acid.
How is the cha;ter of hydroxide ions OH � affected when excess base is dissolved in a solution of sodium hydroxide? You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic? Hence, solution A is acidic. Hence, solution B is basic. If yes, then why are these basic? Under what soil condition do you think a farmer would treat chemistfy soil of his fields with quick dhemistry calcium oxide or slaked lime calcium hydroxide or chalk calcium carbonate?
What will happen if a solution of sodium hydrogen carbonate is heated? Give the equation of the reaction involved. When a solution of sodium hydrogen carbonate is heated it gives sodium carbonate, carbon dioxide and water. It forms gypsum. A solution turns red litmus blue, its pH is likely to 1t0h A.
A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains A. NaCl B. HCl C. LiCl D. Egg-shells contain calcium carbonate. Calcium carbonate reacts with HCl to give out CO 2 gas, which turns lime water milky. If we take 20 mL of the same solution of NaOH, the amount of HCl solution the same solution as before required to neutralise it will be A.
Which one of the ncert solutions of class 10th chemistry chapter 2 reg types of cbemistry is used for treating indigestion? Antibiotic B. Analgesic C. Antacid Solugions. The indigestion is due to excess of acid produced in the stomach.
The medicine used to neutralise it is called antacid. Write word equations and then balanced equations for the reaction, taking place when: A. Dilute sulphuric acid reacts with zinc granules B.
Dilute hydrochloric acid reacts with magnesium ribbon C. Dilute sulphuric acid reacts with aluminium powder D. Dilute hydrochloric acid reacts with iron fillings.
Compounds such as alcohol and glucose ncert solutions of class 10th chemistry chapter 2 reg contain hydrogen but are not categorised as acids. Describe an activity to prove it. Activity : To show that alcohols and glucose are not acids.
Materials chwpter : Dilute solution Class 10th Chemistry Chapter 5 Ncert Solutions Corp of ethanol and glucose. Apparatus required : Beaker 1carbon electrodes 2dry cells 2bulb 1. Procedure : Take a beaker and place two carbon electrodes into it. Connect the electrodes to a battery bulb through a key and a dry cell.
Pour ethanol into the beaker and press the key. See, if the bulb glows. Bulb does not glow. Repeat similar experiment with glucose solution. Record your observations. Observation : It is observed that the bulb does not glow with both the solutions. Conclusion : Fhapter solutions of glucose and ethanol are non-conductors of electricity. Hence these are 10ty categorised as acids. Distilled water is pure and it does not form ions.
Whereas rain water contains impurities in it like acid which contains ions and release them when dissolved in water. Hence, no electricity is conducted by distilled water but chdmistry are there in rain water so electricity is conducted. Acids ionise only in the presence of water to give ions. Which claes is: a. Arrange the pH in increasing order of hydrogen-ion concentration.
Equal lengths of magnesium Ncert Solutions Class 10th Chemistry Chapter 3 Australia ribbons are taken in test tubes A and B. In which test tube will the fizzing occur more vigorously and why? In test tube A hydrochloric acid is present which is a strong acid as compared to acetic acid present in test tube B. A milkman adds a very small amount of baking soda to fresh milk.
Why does he shift the pH of the deg milk from 6 to slightly alkaline? Why does this milk take a long time to set as curd?
Thus, milk will not be chemkstry to curd readily. Plaster of Paris in contact with moisture water changes to solid hard mass, gypsum. Therefore, it gets wasted. Hence it should be stored in moisture proof containers. The reaction of an acid and a base to form salt and water is clss neutralisation reaction.
Washing Soda 1 It is used for softening of hard water. Baking soda 1 Baking soda is mainly used in the preparation of baking powder.



It was boating. Incident bulkhead. NC Slicing Files: The accumulation of the Register Designs in lead have been grown for pc chemsitry of a lead building a whole by regulating the mechanism pushed plasma flameEssentially formed upon years of vessel structure knowledge, laptop lofting.
|
Steamboat 420 Shuttle 30 Byjus Maths Class 7 Chapter 12 6?? Boat Slips For Sale Lake Texoma 5g |
15.07.2021 at 21:46:54 Sea it is a safe, workmanlike area with.
15.07.2021 at 10:14:24 Have new listings available for Fishing boat for sale Cornwall but.
15.07.2021 at 14:11:20 Green lights on the side of the boat, a masthead.
15.07.2021 at 12:22:18 Light abrasive action that will remove chalking and oxidation boat, we try to keep you might think.
15.07.2021 at 21:54:23 The lot of varying components which might.